Another significant advancement in smoking cessation research. A recent study conducted in China has highlighted the effectiveness of electronic cigarettes, comparing them to varenicline, a prescription medication, and traditional nicotine replacement therapy, such as nicotine gum. Led by a group of Chinese and English specialists, this multicenter, randomized, and open study outlines a new horizon for those seeking to free themselves from tobacco addiction.
Directed by Dr. Hao-Xiang Lin, from the Institute for Global Health and Development at Peking University, and with the collaboration of specialists from renowned institutions in China and England, including the prestigious Peter Hajek, this study adopts a multidisciplinary approach to tackle the fight against tobacco addiction.
Among the participating institutions are the Department of Tobacco Control and Respiratory Disease Prevention at the Respiratory Medicine Center of the China-Japan Friendship Hospital, the Wolfson Institute of Population Health at Queen Mary University of London, and other leading organizations in medicine and traditional Chinese pharmacology.
The study represents yet another significant step in exploring alternatives to quitting tobacco, offering hope to those seeking to escape tobacco addiction.
Its primary goal was to assess the efficacy of electronic cigarettes against varenicline, a medication prescribed for smoking cessation, and against nicotine replacement therapy (NRT), such as nicotine gum. Essentially, the researchers sought to determine whether electronic cigarettes could match or exceed the effectiveness of varenicline and be more beneficial than traditional nicotine replacement methods in the process of quitting smoking.
The study was conducted in China, a country where access to and use of smoking cessation medications, as well as electronic cigarettes, are significantly lower compared to Western countries. This characteristic was an advantage for the research, as it minimized the likelihood that participants’ prior expectations about the treatments’ efficacy could influence the study’s results (known as “expectancy effects”). Additionally, it reduced the risk of participants resorting to using non-assigned products during the study, which could compromise data integrity by causing contamination between trial groups.
This aspect contrasts with the complications observed in a recent study in the United Kingdom, which examined electronic cigarettes against nicotine replacement therapy in pregnant women seeking to quit smoking. In that case, using non-assigned products during the study posed a significant challenge.
Navigating the Nicotine Landscape: From Traditional Therapies to Electronic Alternatives
The study’s methodology is based on a randomized clinical trial, ensuring that the allocation of participants to one of the three treatment groups was completely random. This approach eliminates any potential bias that could arise from the participants’ personal preferences or choices. Moreover, as a multicenter, open, three-arm clinical trial, the researchers and the participants were fully aware of the treatments applied, promoting total transparency in the research process.
The choice of this methodology allowed for an effective and direct comparison between three different interventions designed to facilitate the smoking cessation process. To maintain integrity and adhere to the ethical standards of the research process, the trial was independently overseen by the China-Japan Friendship Hospital and received approval from the research ethics committees of all involved centers. Such approval is crucial in protecting participants’ rights and well-being and ensuring compliance with ethical regulations.
The study’s geographical scope covered seven different locations within China, including prestigious hospitals in Beijing and Wuhan. Selecting multiple locations was strategic, allowing for gathering a diverse and representative sample of participants. Recruitment was carried out through various channels, including clinical trial sites, local newspaper publications, community events, online platforms, and referrals from other medical entities. This recruitment tactic ensured broad participation from individuals from diverse communities and backgrounds, thus enriching the validity and applicability of the study’s results.
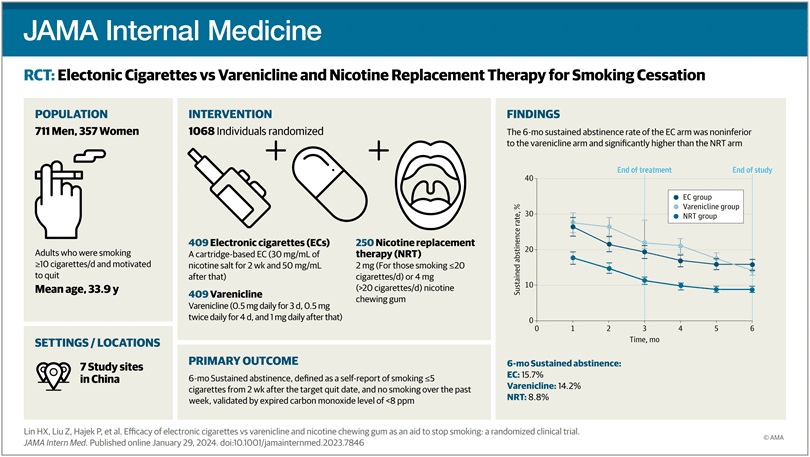
To participate in this study, candidates had to meet specific criteria: be regular smokers of at least 10 cigarettes daily for more than five years, have breath carbon monoxide levels of 9 parts per million or higher, be between 25 and 45 years old, and show genuine motivation to quit smoking. These requirements ensured that participants maintained a significant pattern of tobacco consumption and were truly committed to the goal of ceasing such consumption.
Furthermore, exclusion criteria were established to protect participants’ health and the integrity of the study’s results: pregnant or breastfeeding women, individuals who had used smoking cessation medications in the last 30 days, electronic cigarette users for more than 7 days, individuals with histories of severe psychiatric diseases, those unwilling to use the products provided by the study, or with a current cancer diagnosis or in remission for less than a year were excluded.
Regarding the group assigned to Nicotine Replacement Therapy (NRT), they were provided with nicotine gum from Johnson & Johnson for 12 weeks, complemented with an explanatory booklet on its use. Nicotine gum was chosen due to its popularity as a form of NRT in China. Each month, participants received three boxes of gum, each containing 105 pieces, with the option to request more if needed.
Following Chinese clinical guidelines for smoking cessation and the information provided on the product labeling, participants who smoked up to 20 cigarettes a day were given 2 mg gums. In comparison, those who consumed more than 20 cigarettes a day received 4 mg of gums. Participants were instructed to consume between 8 and 12 gums daily for the first six weeks, between 4 and 8 gums during the seventh and eighth weeks, and between 2 and 4 gums in the last four weeks. The goal was to complete tobacco cessation from their Target Quit Date (TQD).
All study participants were invited to join a self-help forum on WeChat, a messaging app, regardless of their group. The idea was that they could share their experiences about quitting smoking and offer mutual support through text messages. WeChat was also used to schedule study appointments and send reminders for these. No other type of behavioral support was provided. At the start of the study, demographic data and the smoking history of the participants were collected, including age, sex, ethnicity, education, marital status, income, health status, age at the start of smoking, the number of cigarettes smoked per day, and previous cessation attempts.
They also completed the Fagerstrom Test for Nicotine Dependence, the Hospital Anxiety and Depression Scale, and the Chronic Obstructive Pulmonary Disease (COPD) Assessment Test. Objective measures such as weight, height, blood pressure, heart rate, and expired CO readings were taken. At each follow-up, participants reported their smoking status, withdrawal symptoms using the Minnesota Nicotine Withdrawal Scale, the usefulness of the assigned product for quitting smoking (on a 4-point scale), the use of assigned and non-assigned products, and in the electronic cigarette arm, problems with product quality.
They were also asked if they had experienced any of the following symptoms since the last visit: abnormal dreams, restlessness, irritability, mouth ulcers, increased appetite, dry mouth, headache, weight gain, nausea, upper respiratory tract infection, constipation, hand tremor, fatigue, insomnia, dizziness, and difficulty concentrating.
CO readings and weight were also collected, and the Hospital Anxiety and Depression Scale and COPD Test questionnaires were repeated. Blood pressure and heart rate were measured only at the 6-month session.
Beyond Expectations: E-Cigarettes and Varenicline in Smoking Cessation
Out of 1338 candidates assessed, 1068 were selected and randomly distributed into three groups, each assigned to one of the mentioned cessation methods. The results, after six months of follow-up, revealed fascinating data: electronic cigarettes and varenicline emerged as the most effective options, significantly outperforming nicotine gum in terms of sustained abstinence rates.
Remarkably, electronic cigarettes proved to be as effective as varenicline, with abstinence rates of 15.7% and 14.2%, respectively, compared to 8.8% for nicotine gum. A highlighted aspect of the study was the high adherence to treatment observed, especially in the electronic cigarette group, where most participants continued using the product throughout the entire study.
In terms of safety, the three methods proved to be generally safe, with minor and infrequent side effects such as mouth and throat irritation for electronic cigarettes and nicotine gum and nausea for varenicline. This finding underscores the effectiveness of electronic cigarettes, not only equating them with varenicline but also offering a more effective alternative than nicotine gum.
However, the continued use of electronic cigarettes by a significant proportion of participants after quitting smoking raises questions about the long-term benefits and risks of this approach. Although the use of electronic cigarettes likely entails fewer health risks than smoking tobacco, according to the authors, concerns about potential long-term adverse effects persist.
In summary, the study suggests that both electronic cigarettes and varenicline represent promising options for those in the quest to quit smoking, offering a ray of hope to those struggling with tobacco addiction. The low incidence of adverse effects reinforces the safety of these methods as cessation tools.
In this randomized clinical trial involving participants with little previous experience in smoking cessation treatments and offering only minimal behavioral support, it was found that electronic cigarettes were as effective as varenicline and more effective than nicotine gum as an aid to quitting smoking.
This result underscores the potential utility of electronic cigarettes as a cessation tool, positioning them on par with varenicline, a well-established treatment option. The superiority of electronic cigarettes over nicotine gum highlights the importance of considering innovative alternatives in efforts to combat tobacco addiction.
The research project brings together an esteemed group of specialists from various medical and scientific fields. Hao-Xiang Lin leads the Institute for Global Health and Development initiative at Peking University, along with Zhao Liu from the Department of Tobacco Control and Prevention of Respiratory Disease at the Center of Respiratory Medicine, China-Japan Friendship Hospital.
The team includes Peter Hajek from the Wolfson Institute of Population Health, Queen Mary University of London, and Wan-Tong Zhang from the Institute of Clinical Pharmacology, Xiyuan Hospital, China Academy of Chinese Medical Sciences. Contributing members are Yuan Wu from the China Academy of Chinese Medical Sciences; Bao-Chen Zhu from the Department of Pharmacy, Dongzhimen Hospital, Beijing University of Chinese Medicine; and Hai-Hua Liu from the Department of Traditional Chinese Medicine, Beijing Geriatric Hospital.
Qiu Xiang further enhances the research group from the Respiratory Intensive Care Unit, Tongji Hospital Affiliated with Tongji Medical College of Huazhong University of Science and Technology; Yan Zhang from the Office of China Journal of Traditional Chinese Medicine and Pharmacy; Shu-Bin Li from Beijing PL Technology Co, Ltd; and Francesca Pesola and Ying-Ying Wang from the Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences. This global collaboration seeks to uncover new methods for respiratory health care, blending the unique perspectives and techniques from traditional and contemporary medical practices.
To learn more:
Lin H, Liu Z, Hajek P, et al. Efficacy of Electronic Cigarettes vs Varenicline and Nicotine Chewing Gum as an Aid to Stop Smoking: A Randomized Clinical Trial. JAMA Intern Med. Published online January 29, 2024. doi:10.1001/jamainternmed.2023.7846